To the Editor:
Aspirin is a commonly prescribed and ‘over-the-counter’ therapy in older persons. While its use in the secondary prevention of cardiovascular disease (CVD) events is well established1, aspirin is not recommended for primary prevention of CVD in adults aged 60 years or older.2 Low dose aspirin increases the risk of bleeding in older persons3, but whether it has any effect on kidney function is not clear4, 5.
We sought to investigate the effect of low dose aspirin on kidney function in healthy older persons enrolled in the ASPirin in Reducing Events in the Elderly (ASPREE) trial (Clinicaltrials.gov NCT01038583).6 ASPREE was a large double-blind, randomized, placebo-controlled trial designed to assess whether daily treatment with 100 mg of enteric-coated aspirin could extend the duration of life free of dementia and persistent physical disability.
The aims of the present study were to compare the trajectory of kidney function, as measured by estimated glomerular filtration rate (eGFR) or urinary albumin-creatinine ratio (UACR), in participants randomized to aspirin treatment or placebo from the trial’s commencement until its cessation.
In brief, 19,114 healthy community-dwelling individuals aged ≥70 years (aged ≥65 years for African-American and Hispanic participants in the US) were recruited in Australia and in the US. Recruitment took place between March 2010 and December 2014, with annual assessments conducted from randomization until the intervention period ended in June 2017 (median follow-up 4.7 years). Participants were randomly assigned to receive a 100 mg tablet of enteric-coated aspirin or matching placebo daily in double-blind fashion. For this analysis, 7 participants with stage G5 CKD 7 were omitted, as were 1349 participants missing baseline kidney measures. Full details, including the ASPREE trial protocol and main results are reported in detail elsewhere and in the Supplementary Methods (Item S1).6, 8, 9.
Exposure was randomization to aspirin or to placebo. Outcome measures were change in kidney function, assessed as annual decline in eGFR and, separately, annual increase in UACR. No participant was documented as commencing dialysis or receiving a kidney transplant during the intervention period, and participants reaching CKD 5 (eGFR<15) during the trial period were not removed from the analysis.
Linear mixed models were used, which included the group (randomised aspirin versus placebo, i.e. intention to treat), annual visit number (0 [baseline], 1, 2, 3, 4, 5, and 6 years; referred to as “time”), a participant-specific intercept (baseline eGFR or UACR), and a participant-specific slope describing change in eGFR or UACR over time (per annual visit). A treatment-by-time interaction was included to examine whether the trajectory of eGFR or UACR for an average participant differed between the treatment groups. As the distribution of UACR was skewed, it was log (base 2) transformed in all models. To account for dropout due to death, a sensitivity analysis was performed using a shared random effect joint model for longitudinal eGFR or UACR and the overall survival outcome 10. The survival component was modelled using a Cox proportional hazards model, adjusted for baseline age, sex, aspirin, diabetes and time-dependent value of eGFR or UACR.
The primary analysis consisted of 17,758 participants (Figure S1). Baseline characteristics of the participants were well matched across treatment arms (Table 1). The mean age of the cohort was 75.1 years (SD 4.5) and 56.4% were female. The median number of eGFR and UACR measures per patient were 5 (range, 1 to 7) and 4 (range, 1 to 7), respectively. In total 983 deaths occurred among participants included in the primary analysis (523 in the aspirin group, 460 in the placebo group).
Table 1:
Baseline characteristics of the participants randomized to aspirin compared to placebo.
| Placebo N=8938 | Aspirin N=8820 | |
|---|---|---|
| Age at Randomisation, years, mean (SD) | 75.1 (4.5) | 75.2 (4.6) |
| Female Gender, n (%) | 5035 (56.3) | 4987 (56.5) |
| Ethnicity, n (%) | ||
| White/Australia | 7600 (85.0) | 7514 (85.2) |
| White/US | 531 (5.9) | 525 (6.0) |
| Black | 435 (4.9) | 438 (5.0) |
| Hispanic | 236 (2.6) | 227 (2.6) |
| Other/Unknown | 136 (1.5) | 116 (1.3) |
| Country, n (%) | ||
| Australia | 7770 (86.9) | 7653 (86.8) |
| US | 1168 (13.1) | 1167 (13.2) |
| Smoking History, n (%) | ||
| Never Smoked | 4923 (55.1) | 4869 (55.2) |
| Former Smoker | 3655 (40.9) | 3619 (41.0) |
| Current Smoker | 360 (4.0) | 332 (3.8) |
| Alcohol Intake, n (%) | ||
| Current | 6840 (76.5) | 6758 (76.6) |
| Never | 1563 (17.5) | 1528 (17.3) |
| Former | 535 (6.0) | 534 (6.1) |
| Diabetes Mellitus, n (%) | 950 (10.6) | 964 (10.9) |
| Hypertension, n (%) | ||
| No | 2278 (25.5) | 2286 (25.9) |
| Yes, on medication, normal BP | 2208 (24.7) | 2170 (24.6) |
| Yes, on medication, high BP | 2466 (27.6) | 2488 (28.2) |
| Yes, not on medication, high BP | 1986 (22.2) | 1876 (21.3) |
| SBP (mean, mmHg), mean (SD) | 139 (17) | 139 (16) |
| DBP (mean, mmHg), mean (SD) | 77 (10) | 77 (10) |
| Frailty, n (%) | ||
| Not frail | 5246 (58.7) | 5189 (58.8) |
| Pre-frail | 3509 (39.3) | 3425 (38.8) |
| Frail | 183 (2.0) | 206 (2.3) |
| BMI Category, n (%)* | ||
| Underweight <20 kg/m2 | 162 (1.8) | 171 (1.9) |
| Normal 20 - <25 kg/m2 | 2143 (24.1) | 2164 (24.6) |
| Overweight 25 - <30 kg/m2 | 3970 (44.6) | 3864 (44.0) |
| Obese 30+ kg/m2 | 2619 (29.4) | 2587 (29.4) |
| BMI, kg/m2, mean (SD)* | 28.1 (4.7) | 28.1 (4.8) |
| CKD-Epi eGFR (ml/min/m2), mean (SD) | 73.0 (13.9) | 72.9 (14.0) |
| UACR, mg/mmol, median (IQR) | 0.8 (0.5-1.5) | 0.8 (0.5-1.5) |
| eGFR<60 at Baseline, n (%) | 1615 (18.1) | 1637 (18.6) |
| Albuminuria at Baseline, n (%) | 1035 (11.6) | 1010 (11.5) |
SD= standard deviation, IQR= interquartile range, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, eGFR= estimated glomerular filtration rate, UACR= urine albumin creatinine ratio, SBP= systolic blood pressure, DBP= diastolic blood pressure, BMI=body mass index
N=78 missing baseline BMI
Summary measures over time for eGFR and UACR, by treatment assignment, are presented in Figure 1. Results of the mixed models are shown in Table S1 . Mean annual decline in eGFR was not different in participants randomized to aspirin (−0.97 mL/min/1.73 m2, 95% confidence intervals [CI] −1.02, −0.92) compared with those randomized to placebo (−0.99 mL/min/1.73 m2, 95% CI −1.04, −0.94, interaction p-value 0.60). Likewise, annual increase in UACR was similar in participants randomized to aspirin (mean log2 UACR 0.055, 95% CI 0.050, 0.059) compared with placebo (0.051, 95% CI 0.046, 0.056, interaction p-value 0.30). Results of the joint longitudinal and survival models for both outcomes are presented in Table S2. Results were all consistent with the results of the main analysis models, with no evidence of an effect of aspirin treatment on either eGFR decline or UACR increase over time, allowing for loss to follow-up due to mortality.
Figure 1:
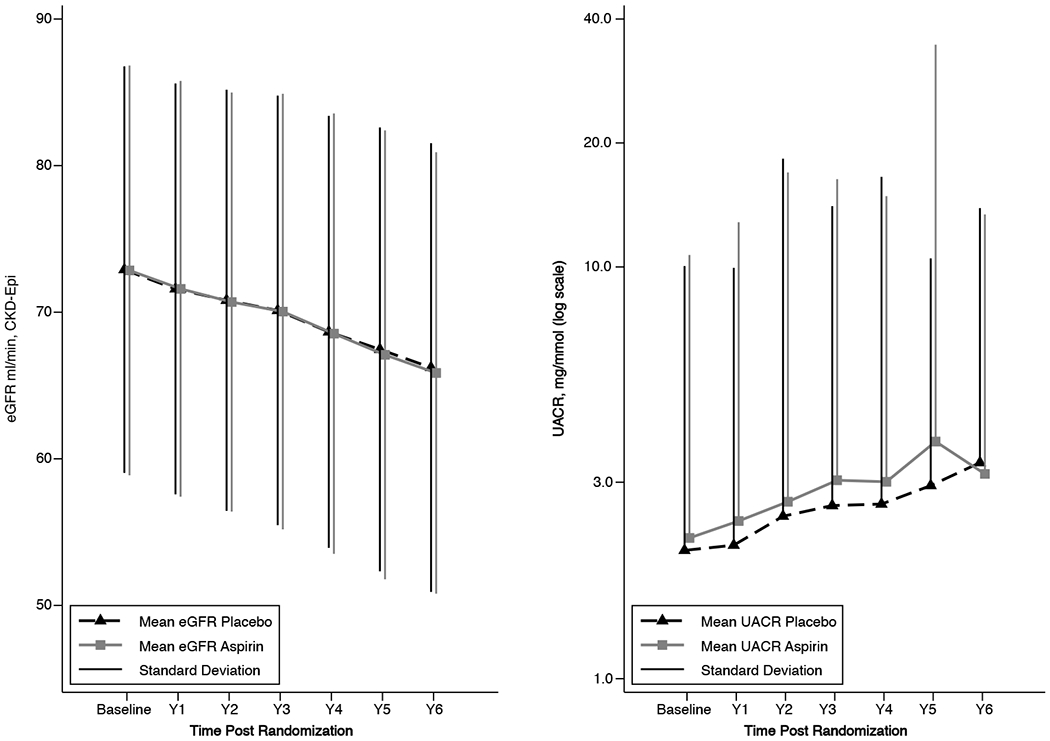
Mean eGFR and UACR by treatment allocation and study visit (error bars represent 1 standard deviation either side of the mean (eGFR) and above the mean (UACR)). Note log scale y-axis for UACR. eGFR, estimated glomerular filtration rate; UACR, urine albumin creatinine ratio.
In summary, we found no evidence of an effect of aspirin on the trajectories of kidney function, as assessed separately by eGFR and UACR, in healthy community-dwelling older persons, over an average of nearly 5 years of follow-up. The results of our study, the largest available trial of older individuals receiving aspirin compared with placebo, suggests that fears over decline in kidney function associated with low-dose aspirin among older individuals may not be justified.
Supplementary Material
Acknowledgements:
We thank the trial staff in Australia and the United States, the participants who volunteered for this trial, and the general practitioners and staff of the medical clinics who cared for the participants.
Support:
ASPREE was supported by the National Institutes of Health National Institute on Aging and the National Cancer Institute (U01AG029824); the National Health and Medical Research Council (NHMRC) of Australia (334047, 1127060); Monash University and the Victorian Cancer Agency.
The work was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (grant numbers U01AG029824, U19AG062682); the National Health and Medical Research Council of Australia (grant numbers 334047, 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia). CMR is supported through a NHMRC Principal Research Fellowship (APP136372). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: CMR declares that he has no other relevant financial interests. The other authors declare that they have no relevant financial interests.
References
- 1.Smith SC, Benjamin EJ, Bonow RO et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011; 58(23): 2432–2446. 10.1016/j.jacc.2011.10.824 [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Aspirin Use to Prevent Cardiovascular Disease: Preventive Medication. 2021; Updated June 9th 2021. Accessed December 14th 2021. https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication#fullrecommendationstart
- 3.McNeil JJ, Wolfe R, Woods RL et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018; 379(16): 1509–1518. 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris RC. Physiologic and pathophysiologic roles of cyclooxygenase-2 in the kidney. Trans Am Clin Climatol Assoc. 2013; 124139–151 [PMC free article] [PubMed] [Google Scholar]
- 5.Violi F, Targher G, Vestri A et al. Effect of aspirin on renal disease progression in patients with type 2 diabetes: A multicenter, double-blind, placebo-controlled, randomized trial. The renaL disEase progression by aspirin in diabetic pAtients (LEDA) trial. Rationale and study design. American Heart Journal. 2017; 189120–127. 10.1016/j.ahj.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 6.McNeil JJ, Woods RL, Nelson MR et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N Engl J Med. 2018; 379(16): 1499–1508. 10.1056/NEJMoa1800722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013; 3(1): 1–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ASPREE IG. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013; 36(2): 555–564. 10.1016/j.cct.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe R, Murray AM, Woods RL et al. The aspirin in reducing events in the elderly trial: Statistical analysis plan. Int J Stroke. 2018; 13(3): 335–338. 10.1177/1747493017741383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics. 2000; 1(4): 465–480. 10.1093/biostatistics/1.4.465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


